Abstract
Polycythemia vera (PV) has high hemoglobin (Hb) concentration caused by clonal proliferation of myeloid cells from a pluripotent stem cell (Prchal, N Engl J Med. 1974). Acquired somatic mutations of JAK2 gene, JAK2V617F and rarely exon 12, are present in virtually all PV patients (Ugo, Nature 2005, Scott N Engl J Med. 2007). The thromboses are the major causes of PV-related morbidity and mortality.
A 38-year female suffered an ischemic stroke in 2017 at age 32 with negative thrombophilia work-up. She had elevated hemoglobin (Hb) 18 g/dL with intermittent elevation of leukocytes and normal platelets with borderline low erythropoietin (EPO) levels. JAK2V617F and exon 12 mutation analysis were negative. Her marrow was interpreted as compatible with PV and normal cytogenetics. Her hematologist treated her with phlebotomies. She was referred to us for an additional evaluation and management. Myeloid next generation sequencing (NGS) revealed a previously undescribed mutation JAK2R715T in the pseudokinase domain (JH2) with allelic burden ~50%. Some of her erythroid progenitors (BFU-Es) grew without extrinsic EPO, a hallmark of PV (Prchal, N Engl J Med. 1974). Phlebotomies were stopped; in two months, her Hb had risen to 17.4 g/dL and hematocrit had changed to 48.5% with normal leukocytes and platelets. We obtained JAK2 inhibitor fedratinib on compassionate basis from the manufacturer. On fedratinib, her Hb normalized to 15.2 g/dL with 42.8 % hematocrit. Her iron deficiency was corrected without increase of her Hb. The patient's germline mutation was confirmed by DNA sequencing of her nails. We encouraged her to have her only son and parents tested by NGS. The same JAK2R715T mutation was found in her 10-year-old son's blood sample. He also has elevated Hb at 16.2 g/dL (normal for age 11.5-15.5 g/dL), normal leukocytes and platelets were 400 K/µl. Her asymptomatic 63-year-old mother’ Hb was 17 g/dL, hematocrit 50.5%, and sequencing of her JAK2 also revealed JAK2R715T at ~50%.
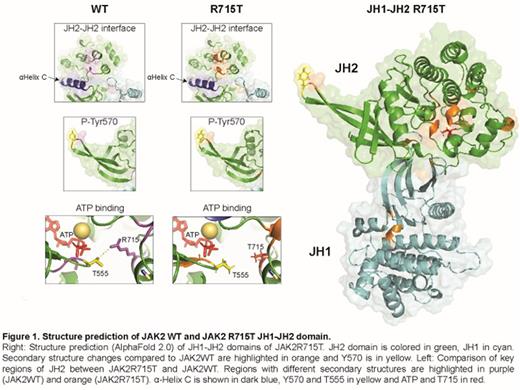
To assess how the R715T mutation may affect kinase activation, the JH1-JH2 domains of JAK2R715T and JAK2WT were modelled using AlphaFold 2.0 (Jumper, Nature 2021). The overall structure of JAK2R715T JH2 was more rigid than that of JAK2WT, in agreement with DynaMut in silico prediction (PMID: 29718330). -Helix C is shown in dark blue, Y570 and T555 in yellow, and ATP and T715 in red. Detailed comparison of JAK2R715T with JAK2WT JH2 (see Figure) indicated that the rigidification of the structure involved notably residues of the JH2-JH2 trans-dimerization domain centered around -Helix C. This has been suggested to mediate direct JH2-JH2 contacts (Wilmes, Science 2020) and a lengthening of the ß-sheet region surrounding the inhibitory phosphorylation site Tyr570 (Echarri, Mol Cell Biol. 2004). The R715 is in direct vicinity of the ATP binding pocket and promotes a closed lobe conformation through interaction with Thr555 of the nucleotide-binding loop (Bandaranayake, Nat Struct Mol Biol. 2012). We suggest that loss of this interaction may result in a more opened lobe conformation and disturbing of ATP binding to JH2, which regulates JAK2 kinase activity (Hammarén, Proc Natl Acad Sci USA 2015).
To evaluate the effect of JAK2R715T in erythroid progenitor cells, the patient's erythroid progenitors derived from peripheral mononuclear cells were grown for 21 days with and without JAK inhibitor (ruxolitinib) following the previously published in vitro expansion protocol (Bruchova, Blood Cells Mol Dis, 2009). Compared to PVs and healthy controls, the patient cells showed increased proliferation. JAK inhibitor ruxolitinib treatment of patient cells induced decreased proliferation and differentiation compared to untreated cells. Assessment of STAT5 phosphorylation on patient's blood cells confirmed increased JAK2 activity to levels even higher than this of patients with confirmed JAK2V617F positive PV. X-chromosome based clonality studies of both females are ongoing. The EPO-independent colonies of her BFU-E is being evaluated for possible acquired conversion to homozygosity by uniparental disomy that is present in PV (Kralovics, Experimental Hematology, 2002).
We conclude that this is the first example of dominantly inherited PV phenotype in 3 generations due to a novel germline JAK2R715T gain-of function mutation.
Disclosures
Constantinescu:MyeloPro Research and Diagnostics GmbH, Vienna, Austria: Current Employment, Other: Co-founder.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal